Cracking the Molecular Code of Opioid Addiction: From Humans to Rodents
- Olivier George
- 2 days ago
- 3 min read

Opioid use disorder (OUD) has grown into a severe global health emergency, yet long term abstinence remains an elusive goal for many. One major reason is that chronic opioid use leaves lasting marks on the brain that persist long after someone stops using. In our latest study, we used advanced genetic sequencing to compare the molecular changes in the human brain with those in animal models to find common "signatures" of addiction.
The Big Question
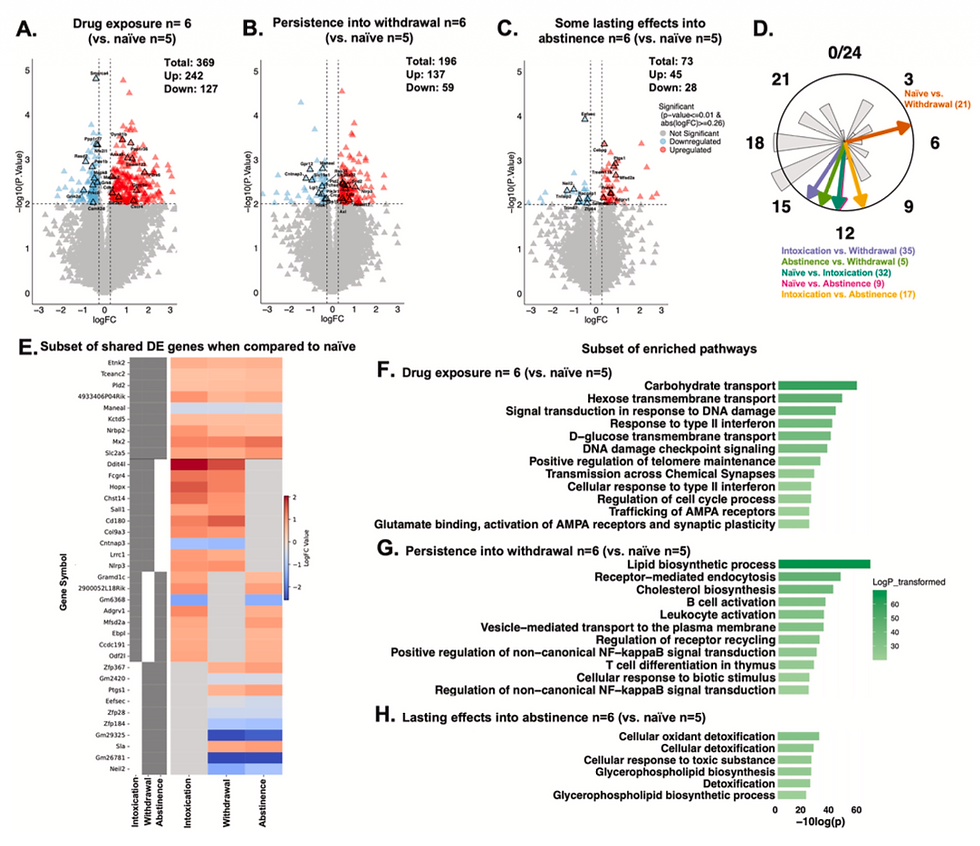
We wanted to know if the changes in gene activity that occur in humans with OUD are mirrored in the brains of mice and rats. In collaboration with the Logan Lab, we focused on the nucleus accumbens (NAc), a key part of the brain's reward and motivation circuit. We also set out to determine if these molecular signatures differ between males and females, and whether they change depending on whether the drug use was "voluntary" (self administered) or "forced."
The Study: A Cross-Species Comparison
To find these answers, we performed RNA sequencing on postmortem human tissue from individuals with OUD and compared them to age and sex matched controls. We then conducted similar sequencing in mouse and rat models. This allowed us to look across species at different stages of the addiction cycle:
Acute use: Short term exposure.
Prolonged withdrawal: Long term abstinence.
Volitional vs. Non-volitional: Comparing subjects that chose to take the drug versus those that were given it passively.
What We Discovered
Our findings revealed a striking and complex molecular landscape:
Sex Matters Most: We found a very strong "molecular concordance" (a shared signature) between the NAc of females with OUD and female rodents. This shared signature was much stronger in females than in males.
Opposite Signatures: Interestingly, we discovered that males and females often show opposite changes in gene expression in the NAc. A gene that is "turned up" in a female with OUD might be "turned down" in a male.
The Power of Choice: Rodent models where the animals voluntarily self administered opioids showed a much closer molecular match to the human condition than models with forced exposure.
A Signature of Abstinence: We identified a specific group of genes involved in "extracellular matrix remodeling" (how the space between brain cells is structured) that was consistently altered across species during long term abstinence.
Why It Matters
This research is a major step forward for translational medicine. It proves that while rodent models are excellent tools, we must be incredibly careful to use models that include both sexes and volitional behavior to truly capture the human experience of OUD.
By identifying the specific genes and pathways that are shared between humans and rodents—especially the unique signatures found in females—we can begin to develop more targeted, sex specific treatments. We believe that understanding these shared biological blueprints is the key to creating medications that can effectively "reset" the brain and support lasting recovery for everyone.
Reference: Shelton, M. A., Horan, N., Xue, X., Maturin, L., Eacret, D., Michaud, J., Singh, N., Williams, B. R., Gamble, M. C., Seggio, J. A., Fish, M. K., Phan, B. N., Tseng, G. C., Blendy, J. A., Solberg Woods, L. C., Palmer, A. A., George, O., Logan, R. W., & Seney, M. L. (2025). Sex-Specific Concordance of Striatal Transcriptional Signatures of Opioid Addiction in Human and Rodent Brains. Biological Psychiatry Global Open Science. https://www.sciencedirect.com/science/article/pii/S2667174325000308?via%3Dihub





Comments